Irritable bowel syndrome. Celiac disease. Gastroesophageal reflux disease. Ulcerative colitis.
If you’ve ever suffered gut issues, you’ll be familiar with the frustrating merry-go-round you step on to find a definitive diagnosis of your condition.
But a new gut wearable by New Zealand-based medtech startup Alimetry is hoping to transform the process.
The Gastric Alimetry system uses AI and printed electronics to measure and interpret the gut’s electrical signals, providing accurate analysis of gut disorders that have previously been challenging to diagnose using conventional testing.
Doctor Greg O’Grady, Alimetry’s chief executive officer and professor of surgery at the University of Auckland, told Information Age, “the clinical need for the product is what’s driven its genesis.”
As a surgeon and a specialist in intestinal disease, he said he sees a huge number of patients who never receive a satisfactory explanation for their symptoms.
Chronic gut issues plague 40 per cent of the world’s population, and digestive concerns are among the top ten most common complaints reported to Australian GPs.
Gut ailments can have a range of causes, including diet, disease, stress and issues relating to the gut-brain axis, and symptoms can include bloating, indigestion, nausea, heartburn and irregular bowel movements.
The technology will soon be rolled out to the US market following approval for clinical use from the US Food and Drug Administration (FDA).
More than 40 clinics and hospitals across the globe have already signed up to use the device, although it is yet to be approved for clinical use in Australia.
High-tech digest test bests the rest
The Alimetry system works using a process called Body Surface Gastric Mapping, which measures electrical signals created by movements in the gut.
This operates much like an electrocardiogram (ECG) or electroencephalogram (EEG) but requires far higher sensitivity, as the gut’s electrical signals are around 100 times weaker than those created by the heart or brain and can easily be obscured by other impulses, such as those made by the abdominal wall.
Professor Gerald Holtmann, director of gastroenterology and hepatology at Brisbane’s Princess Alexandra Hospital and professor of medicine at the University of Queensland, pointed out researchers have been attempting to measure the stomach’s electrical signals for over 100 years, but until now have been unable to differentiate them from other impulses.
“It is an old technology when it comes to the underlying principles, and I think it's valid,” he explained.
Patients using the device are asked to eat and digest a meal while manually logging their symptoms into an app.
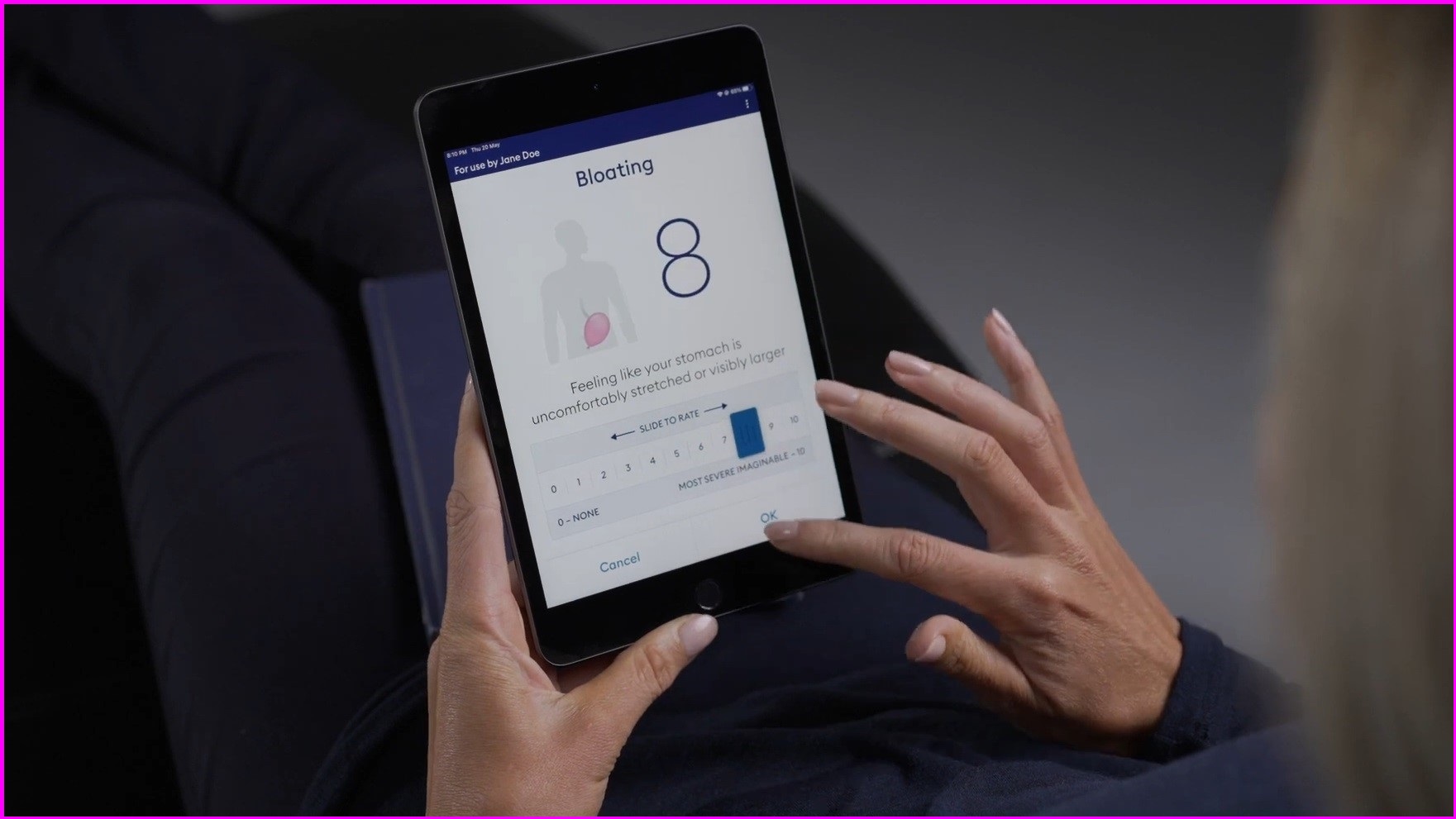
Users are asked to log their symptoms. Photo: Alimetry
The recorded data is then sent to the cloud for AI analysis before a report is compiled and sent to the clinician.
O’Grady explained the system consists of several technologies working in tandem.
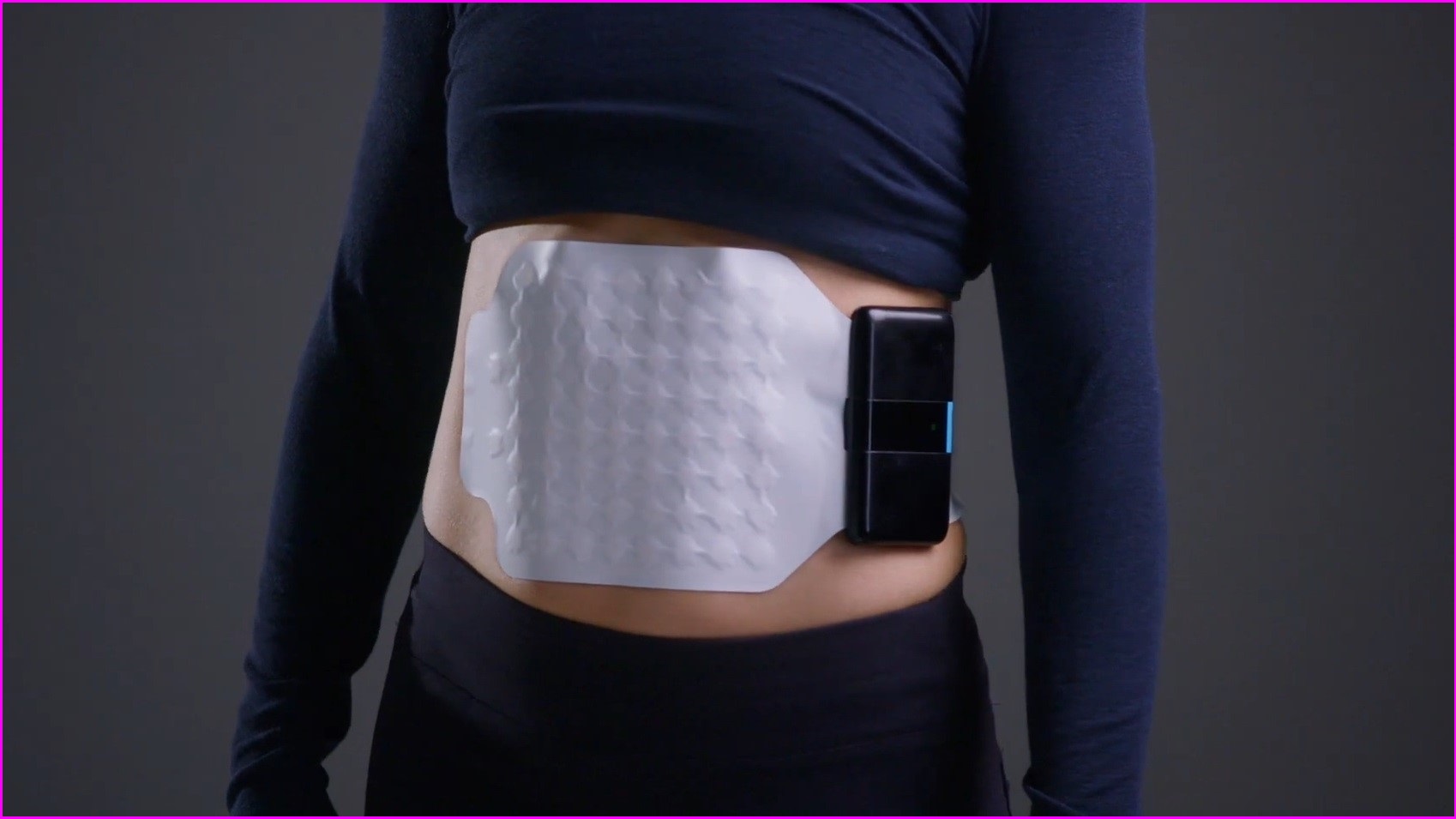
“It has a reader device, which is kind of the hardware and the firmware — that sits on the patient, it's wearable — and then there's an adhesive patch of electrodes, and that's single use.”
The patch is a printed electronic, where conductive inks are printed onto a very thin piece of plastic.
O’Grady said this means they can fit a larger number of electrodes in a small area, making it easier to pick up the gut’s weaker signals.
Another key was the use of AI, which is becoming increasingly popular in medical settings.
The Alimetry device uses AI-powered neural networks trained on thousands of test cases to analyse and interpret data, an innovation the company has only just rolled out.
“Before that, we just had to use traditional filtering algorithms, but the neural network performance is dramatically better,” added O’Grady.
“We're very excited about eliminating noise to the point where we could almost have the patient walking around with the device at home rather than being in the clinic.”
A solution that’s easy to stomach
The Alimetry device has already been used in Australia in a range of trials, but O’Grady said it’s unlikely to be available for clinical use until at least 2025.
Holtmann said while more research is required, the initial reports look good.
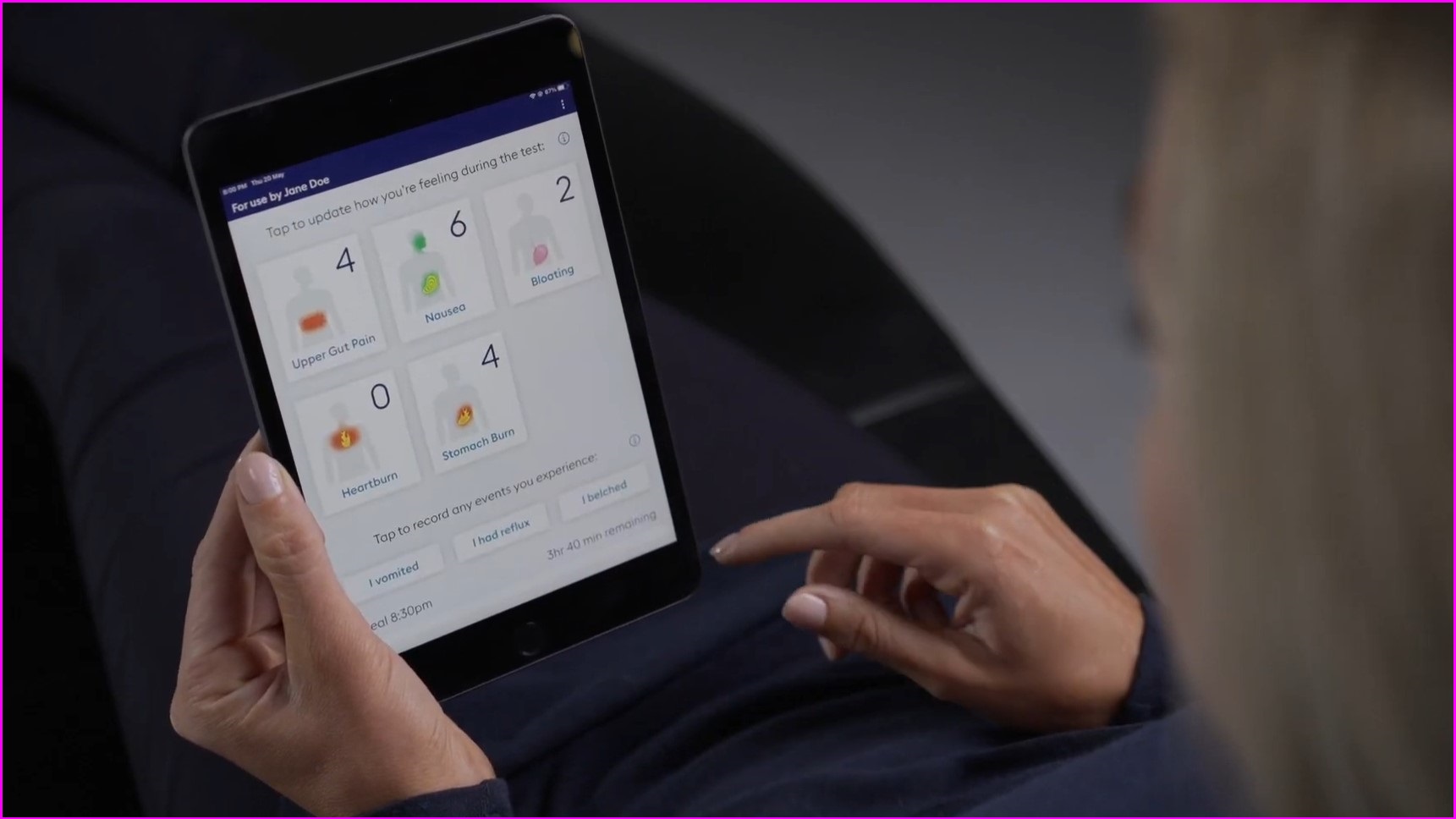
Any adverse reactions can be noted in the app. Photo: Alimetry
He added the current diagnostic process can be challenging for patients, including months or years of invasive tests, often reaching inconclusive results.
O’Grady agreed, sharing that some patients can spend upwards of five years on a diagnostic odyssey trying to get a diagnosis.
“With this test, we’re really trying to rule things in with a single test and say for sure whether the patient’s symptoms are coming from their stomach or their nerves or somewhere else, like the gut-brain axis,” he shared.
The device also offers a low-cost, low-risk option, which Holtmann said makes it attractive to providers looking to help the many patients seeking answers.
“There's a really huge unmet need, and therefore the risk-benefit ratio is great.”
The company is already exploring the device’s potential for use in other health areas, such as colonic disorders, and is particularly motivated to move into paediatrics.
“Parents then don't have to go through the stress of putting their children under anaesthesia to have a scope or expose them to radiation when they're still growing,” said O’Grady.
He added he hopes the Alimetry device will spark a new wave of gut health wearables, filling what is currently “a hollow space in the field”.
“We've got wearables for lots of other organs, and people are really curious about what happens when they eat different diets or try different drugs.
“We're hoping that this product will be kind of a pioneering product for a whole new category of GI monitoring devices and wearables.”